Now we need to focus on benzene substituents and how they affect the location of subsequent additions. Here’s a list of the ones you would most likely see:
Generally speaking, electron donators / activators have a lone pair of electrons or an electron density that “pushes” into the benzene. Electron withdrawers / deactivators have a positive charge on the substituent or a very electronegative atom attached to it, which “pulls” electrons out of the benzene.
All activators AND halogens are ortho-para directors; Deactivators (not halogens) are meta-directing.
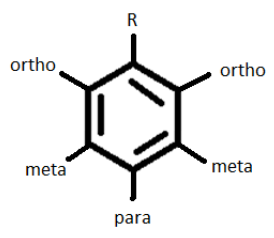
Therefore, depending on the character of the initial substituent (R), a subsequent substituent would be placed at the ortho or para position if R is an activator/halogen or at the meta position if it is a deactivator (but not a halogen).
Other facts to know:
- The more electron donating groups a benzene ring has initially, the faster an EAS reaction will occur (due to increased electron density to make benzene a better nucleophile).
- If there are already two or more substituents on the ring, the strongest donating group gets priority when choosing the location of the added substituent.
- When given an ortho / para choice, substituents will go to the location with the least steric strain.
Get everything you need in one place. Start studying today for free.







.jpg)










